Body Centred Cubic Cell
In each cubic unit cell there are 8 atoms at the corners. The unit cell is defined as the smallest repeating unit having the full symmetry of the crystal structure.

Comparison Of Space Filling In Different Cubic Structures Unit Cell Material Science Physical Chemistry
After clamping the heart mice were placed on ice to lower their body temperature and to allow for gel formation.

. 111 Silver crystallises in fcc lattice. ASCII characters only characters found on a standard US keyboard. So the contribution of one atom to a unit cell is 18.
110 Calculate the efficiency of packing in case of a metal crystal for i simple cubic ii body centred cubic and iii face centred cubic with the assumptions that atoms are touching each other. There are three main varieties of these crystals. It is the percentage of total space filled by the particles.
Simple cubic P Body- centred cubic I Face centered cubic F Bravais Lattice in Two dimension-Plane lattice. In the face-centred cubic unit cell octahedral voids are located at the body centre and all the edge centres of the cube. An atom at the corner is equally shared by 8 unit cells.
A Cubic perovskite crystal structureFor photovoltaically interesting perovskites the large cation A is usually the methylammonium ion CH 3 NH 3 the small cation B is Pb and the anion X is a. Crystal structure is described in terms of the geometry of arrangement of particles in the unit cells. Must contain at least 4 different symbols.
The portions of the unsectioned brains ipsilateral and contralateral sensory-motor cortex 23 mm thick were cleared using the advanced CUBIC protocol 15 and imaged in 3D using a confocal microscope C2 plus Nikon. The geometry of the unit cell is defined as a parallelepiped providing six lattice parameters taken as the lengths of the cell edges a b c and the angles between them α β γ. Volume of atoms in unit cell In a simple cubic structure the atoms occupies at the eight corners.
Body-centred Cubic Unit Cell BCC A BCC unit cell has atoms at each corner of the cube and an atom at the centre of the structure. 8 18 1 atom. The diagram shown below is an open structure.
6 to 30 characters long. Body-centered cubic abbreviated cI or bcc. This is a typical lattice structure where the atomic planes or lattice planes lie within the gaps of the lower planes of the respective atoms.
Therefore the total number of atoms in one unit cell is. In this type the unit cell is shaped like a cube. In the cubic close packing ccp or fcc face-centred cubic unit cell lattice the constituents particles atoms molecules or ions are located at the corner of the unit cell as well as at the face-centred.
In crystallography the cubic or isometric crystal system is a crystal system where the unit cell is in the shape of a cubeThis is one of the most common and simplest shapes found in crystals and minerals. Primitive cubic abbreviated cP and alternatively called simple cubic. It means that one atom is directly in contact with eight other atoms or lattice points.
Body-Centred Cubic BCC Lattice structure.

Cubic Lattice From Wolfram Mathworld Cell Forms Unit Cell Lattice

Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell The Unit Atom
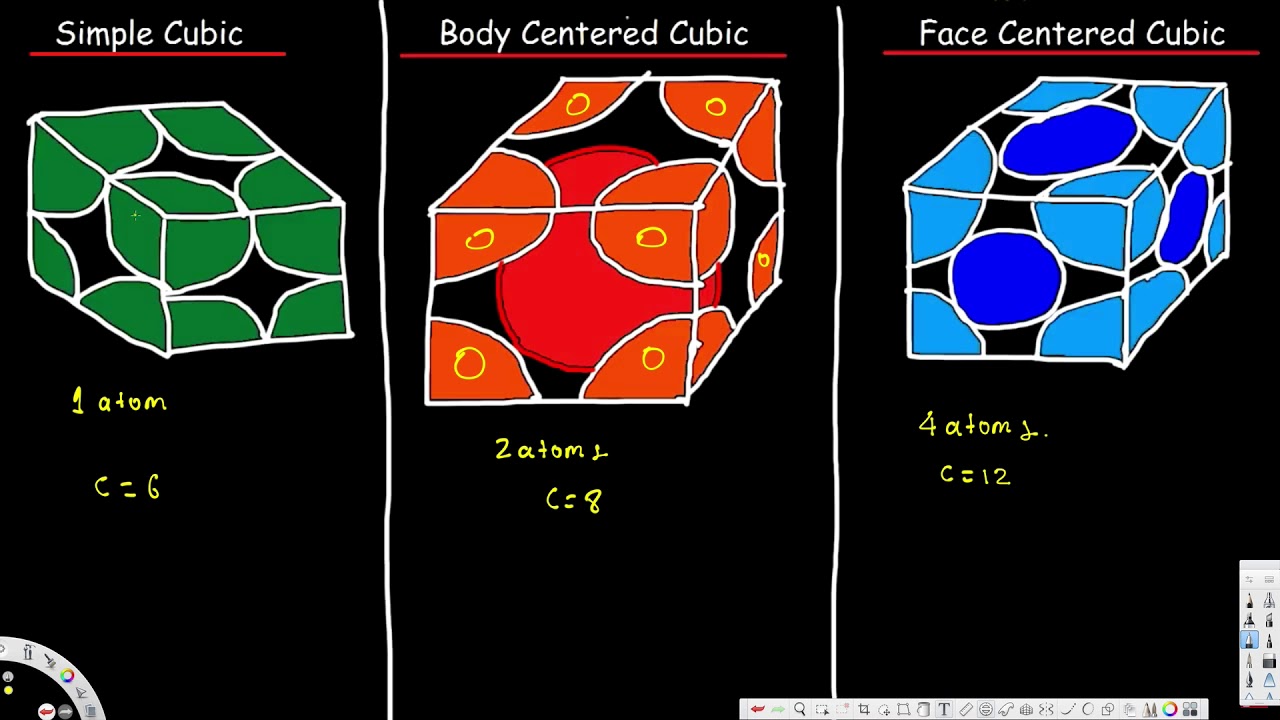
Unit Cell Simple Cubic Body Centered Cubic Face Centered Cubic Cryst Unit Cell Nomenclature Chemistry Crystal Lattice Structure

Unit Cell Body Centered Cubic Crystal Lattice Structures Physical E Unit Cell Crystal Lattice Structure Nomenclature Chemistry
Comments
Post a Comment